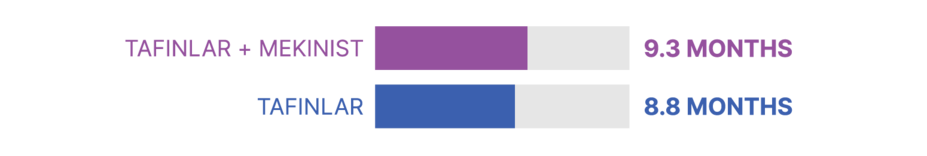What is TAFINLAR + MEKINIST?
.
TAFINLAR + MEKINIST
has received 6 approvals and has treated more than 277,000 patients across indications since 2013
has had efficacy and safety established in clinical trials evaluating more than 3,200 patients across certain tumor types
TAFINLAR + MEKINIST is a combination therapy for BRAF+ melanoma
It is a targeted therapy that can be used to treat 2 different stages of BRAF+ melanoma†:
Adjuvant (after surgical removal) treatment of BRAF+ melanoma, to help prevent your melanoma from coming back after surgery
Treatment of metastatic (has spread to other parts of the body) melanoma or unresectable (cannot be removed by surgery) melanoma
*Based on IQVIA data in the US from January 2014 to January 2024.
†TAFINLAR + MEKINIST should not be used to treat people with a type of skin cancer called wild-type BRAF melanoma. MEKINIST should not be used to treat people who already have received a BRAF inhibitor for treatment of their melanoma and it did not work or is no longer working. In clinical studies, patients with BRAF+ melanoma had either the V600E or V600K mutation.
How does TAFINLAR + MEKINIST work?
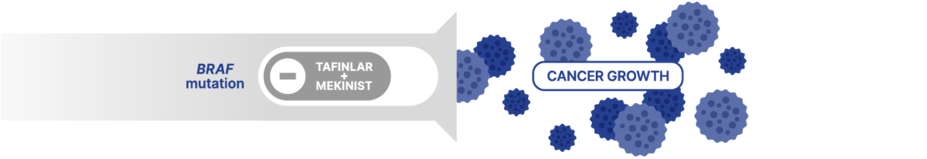
Some cancers express proteins called BRAF and MEK, two distinct points on something called the MAPK pathway, that control cancer growth.
If the BRAF protein becomes mutated, it starts working incorrectly and flips on a switch that causes continuous production of the protein, which can grow uncontrollably.
TAFINLAR + MEKINIST is a combination treatment that targets this switch and may help slow the growth of cancer.
BRAF mutations are found in up to 50% of patients with certain types of cancers
They are typically acquired changes, which means they develop sometime after birth. Acquired changes are not genetic, so the mutation won't be passed down to children.
What kind of treatment is TAFINLAR + MEKINIST?
As a targeted combination therapy, TAFINLAR + MEKINIST helps inhibit out-of-control cell growth by blocking cell-growth signals associated with the BRAF gene. TAFINLAR and MEKINIST work together, with each targeting a different point in the cell-signaling process. Although TAFINLAR + MEKINIST targets specific cancer cells, it may also affect healthy cells in the body.
TAFINLAR + MEKINIST is not a chemotherapy. It is an oral therapy, so you will not need to travel to an infusion center to receive treatment.
TAFINLAR + MEKINIST offers the strength of targeted treatment
Results after surgery
TAFINLAR + MEKINIST lowered the chance of cancer returning after surgery
TAFINLAR + MEKINIST is a targeted therapy that can be used as adjuvant (after surgery) treatment of BRAF+ melanoma.
Adjuvant treatment is additional therapy given after a primary treatment (such as surgery) to further decrease the risk that the disease could progress. Your health care provider may prescribe you TAFINLAR + MEKINIST to help prevent your melanoma from coming back after surgery.
TAFINLAR + MEKINIST was studied in patients with stage III BRAF+ melanoma previously removed by surgery. Eight hundred seventy adult patients were treated with a combination of TAFINLAR + MEKINIST or placebo for 12 months. See the results of this study below.
Benefits of TAFINLAR + MEKINIST therapy after surgery
The risk of cancer returning after surgery was reduced by nearly 50% at 5 years.*
49% risk reduction vs placebo.
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
Remaining relapse free after surgery
More patients treated with TAFINLAR + MEKINIST remained relapse free after 12 months of treatment.
At first check-in, 62% of patients treated with TAFINLAR + MEKINIST were cancer free, compared with 43% with placebo (sugar pill).
At 5 years, 52% of patients treated with TAFINLAR + MEKINIST were cancer free, compared with 36% with placebo (sugar pill).
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
No spreading to distant body parts (metastases)
More patients treated with TAFINLAR + MEKINIST also saw their cancer had not spread to distant (meaning other) parts of their bodies at 5 years compared with placebo.* When cancer spreads near the original site, surgery is still an option. Distant spreading, however, is associated with lower survival. When cancer does not spread to other parts of the body, it is known as distant metastasis-free survival (DMFS).
Metastases free at 5 years
65% saw no cancer in distant parts of their bodies | 54% saw no cancer in distant parts of their bodies |
65% of patients treated with TAFINLAR + MEKINIST saw no cancer in distant parts of their bodies after 5 years, compared with 54% of patients on placebo (sugar pill).
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
*As demonstrated by a clinical trial that included 870 patients with BRAF V600E or V600K mutation-positive stage III melanoma that was previously removed by surgery. Patients were treated with a combination of TAFINLAR + MEKINIST or placebo for 12 months. Patients were followed for an average of 5 years after starting treatment. These numbers are based on the 5-year check-in.
Results for advanced melanoma
TAFINLAR + MEKINIST for advanced melanoma may offer a chance to extend your life (known as overall survival), as seen in 2 studies
TAFINLAR + MEKINIST is a targeted therapy that can be used as treatment for BRAF+ metastatic or inoperable melanoma.
Your health care provider may prescribe you TAFINLAR + MEKINIST to help slow the progression of your melanoma if you have tested positive for BRAF melanoma.
TAFINLAR + MEKINIST was studied in patients with advanced (unresectable or metastatic) BRAF+ melanoma in 2 studies. In Study 1, 704 adult patients were treated with a combination of TAFINLAR + MEKINIST or vemurafenib (another cancer drug) alone. In Study 2, 423 patients with advanced BRAF+ melanoma were treated with a combination of TAFINLAR + MEKINIST or TAFINLAR alone. See the results of these studies below.
The possibility of extending life
The first analysis of Study 1 happened in April 2014, when about half of the patients receiving TAFINLAR + MEKINIST had 11 months of follow-up.
In this analysis, 72% of patients receiving TAFINLAR + MEKINIST were alive vs 65% of patients receiving vemurafenib
TAFINLAR + MEKINIST reduced the risk of death by 31% compared with vemurafenib
A second analysis of Study 1 happened once 5 years of follow-up data had been collected.
This analysis reported that 36% of patients receiving TAFINLAR + MEKINIST were still alive at 5 years vs 23% of patients receiving vemurafenib
This analysis was not pre-planned to detect a false positive or show a difference between treatments
Living without progression (known as progression-free survival)
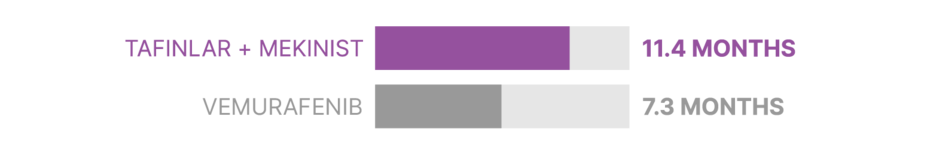
The first analysis of Study 1 reported that TAFINLAR + MEKINIST reduced the risk of disease progression or death by 44% compared with vemurafenib. About half of patients taking each treatment were living without their disease worsening after:
In Study 1, about half of patients taking TAFINLAR + MEKINIST were living without their disease worsening at 11.4 months, compared with 7.3 months for patients taking vemurafenib.
The second analysis of Study 1 found that 20% of patients receiving TAFINLAR + MEKINIST were living without their disease worsening at 5 years vs 9% of patients on vemurafenib.
This analysis was not pre-planned to detect a false positive or show a difference between treatments
Study 2 also looked at how long patients lived without their disease worsening. In this study, which included 423 patients, about half of patients taking each treatment were living without their disease worsening after:
In Study 2, about half of patients taking TAFINLAR + MEKINIST were living without their disease worsening at 9.3 months compared with 8.8 months for patients taking TAFINLAR alone.
A pooled analysis of Study 1 and Study 2 was conducted as well. This analysis included 563 adult patients with BRAF V600+ advanced melanoma who were treated with a combination of TAFINLAR + MEKINIST. See the results of this pooled analysis below.
At 5 years, nearly a fifth of patients taking TAFINLAR + MEKINIST were living without their disease worsening
Nearly 1 in 5 patients was living progression free at 5 years in the pooled analysis of Study 1 and Study 2.
This analysis was not pre-planned to detect a false positive or show a difference between treatments.
More patients had their tumors shrink or disappear
In Study 2, more patients had their tumors shrink or disappear (known as the overall response rate) with TAFINLAR + MEKINIST.
66% overall response rate | 51% overall response rate |
Patients taking TAFINLAR + MEKINIST saw a 66% overall response rate. Patients taking TAFINLAR alone saw a 51% overall response rate.
Of patients taking TAFINLAR + MEKINIST, 10% saw their disease disappear and 56% saw their disease shrink. Of patients taking TAFINLAR alone, 8% saw their disease disappear and 42% saw their disease shrink.
TAFINLAR + MEKINIST is effective in advanced melanoma with brain metastases
Metastatic melanoma that travels to the brain is one of the most severe manifestations of melanoma. It is difficult to treat and is often given a poor prognosis. TAFINLAR + MEKINIST was studied in 121 adult patients with BRAF V600E/K mutation–positive melanoma that had spread to the brain. Patients were treated with TAFINLAR + MEKINIST.
In this clinical study of patients who had their melanoma spread to the brain and were treated with TAFINLAR + MEKINIST, 50% had their brain tumors shrink or disappear (known as an intracranial response). This included 4.1% of patients whose brain tumors disappeared and 46% of patients whose brain tumors shrank.
Of the patients who had an intracranial response, about half did not have their brain tumors progress for at least 6.4 months.
Taking TAFINLAR + MEKINIST
Learn about dosing and administration