What is the BRAF V600E mutation?
Receiving a cancer diagnosis is overwhelming and comes with a lot of information to take in. Let’s look at the essential things you should know about low-grade glioma (LGG) with a BRAF V600E mutation in children. |
The basics
LGG is the most common brain tumor diagnosed in children. These tumors are typically removed through surgery and treated with chemotherapy if needed. Some children, however, may have a damaged BRAF gene—known as a mutation—that may cause the tumor to act more aggressively. | |
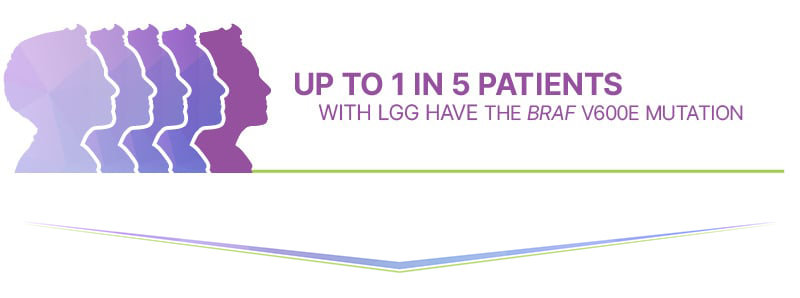
Specifically, the BRAF V600E mutation is the second most common mutation found in LGG. |
Why does the BRAF V600E mutation matter?
LGG tumors with a BRAF V600E mutation are stubborn and often return and continue to grow (progress), even after successful surgery. These specific tumors may also respond poorly to chemotherapy—the most frequently used treatment following surgery. | |
After the tumor is removed, doctors will send a sample of it to a genetic laboratory, where its DNA will be analyzed to see if it contains a BRAF mutation. A positive result means that the BRAF V600E mutation is present. |