Results With TAFINLAR + MEKINIST
Proven clinical trial results
In a clinical trial, TAFINLAR + MEKINIST was studied in 2 different groups of people with the BRAF V600E genetic mutation: those taking TAFINLAR + MEKINIST as their first treatment and those previously treated before taking TAFINLAR + MEKINIST.
TAFINLAR + MEKINIST is a proven choice for patients with BRAF V600E metastatic NSCLC
Slows or shrinks tumors
In a clinical trial, TAFINLAR + MEKINIST was shown to help shrink or slow the growth of tumors in previously untreated and previously treated patients.
Overall response rate
The overall response rate measures the size or number of tumors people have. This number includes:
people whose tumors became smaller or fewer in number (which is called a partial response), and
people whose tumors disappeared completely (which is called a complete response); a complete response is not the same thing as a cure
For those taking TAFINLAR + MEKINIST as their first treatment
A total of 61% of 36 untreated patients achieved a response with TAFINLAR + MEKINIST. A total of 8% of untreated patients had a complete response and 53% had a partial response.
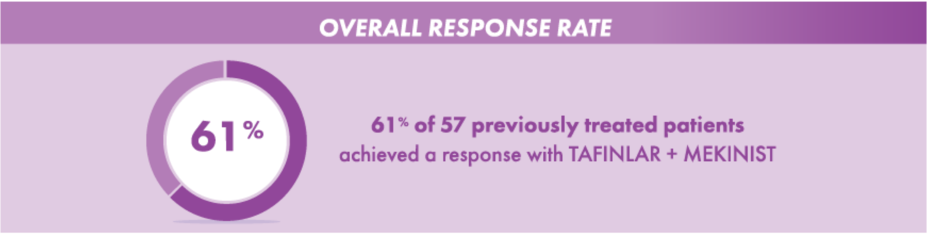
For those previously treated before taking TAFINLAR + MEKINIST
A total of 61% of 57 previously treated patients achieved a response with TAFINLAR + MEKINIST. Of these responders, 5% had a complete response and 56% of patients had a partial response.
Duration of response
Duration of response is the length of time that a tumor continues to respond to treatment without the cancer growing or spreading
For those taking TAFINLAR + MEKINIST as their first treatment
The median (or midpoint) duration of response for patients who have never been treated was 15.2 months. That means that half of the patients who responded to treatment continued to respond longer than 15.2 months and half responded for less than 15.2 months.
For those previously treated before taking TAFINLAR + MEKINIST
The median (or midpoint) duration of response for patients who were previously treated was 9 months. That means that half of the patients who responded to treatment continued to respond longer than 9 months and half responded for less than 9 months.